Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
JNC TN8400 2001-008, 36 Pages, 2001/03
Research on geologic disposal of high-level radioactive waste(HLW) has been underway in many countries. Bentonite exhibiting a low permeability, high swelling property and high sorption capacity for many radioelements is proposed as a buffer material in many countlies. Recently, cementitious materials are considered as candidate matelials for the geologic disposal of high-level radioactive waste. As the pH and the Ca, Na, K contents of hyperalkaline pore water from the cementitious materials are high, this hyperalkaline pore water would alter the buffer material. The main aim of this study is to investigate the effect of alkaline pore water into the bentonite. Used materials are montmorillonite, albite and quartz composing bentonite. These minerals mixed in a constant ratio (1:1wt%) made to react to distilled water and the alkali solutions (pH11-13). These studies have been conducted at temperatures of 50 - 150 C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test
C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test  Montmorillonite test
Montmorillonite test  Montmorillonite and quartz mixing test Activation energies (E
Montmorillonite and quartz mixing test Activation energies (E ) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
Isogai, Takeshi*; Oda, Chie
JNC TN8400 2000-025, 48 Pages, 2000/09
Porewater chemistly in compacted bentonite would affect a performance of engineered barrier system in a high-level radioactive waste repository, whereas there are little information of the porewater based on experimental data. The previous study provided a new method of direct pH measurement for highly compacted bentonite system and demonstrated some tests for compacted bentonite samples (the dry densities: 1.6 [g/cm ] and 1.8 [g/cm
] and 1.8 [g/cm ]) both with the de-ionized water and with the NaCl solution. In this study, the solution equilibrated with low alkalinity cement were used in the direct pH measurement to see the effect of the composition of the external solutions, in which the bentonite column immersed. The result showed that the pH value of porewater in the cementitious condition was around 9 during the immersed time 1 to 3 months, while after 6 months became the porewater pH 10.6, which was equal to pH of the external solution.
]) both with the de-ionized water and with the NaCl solution. In this study, the solution equilibrated with low alkalinity cement were used in the direct pH measurement to see the effect of the composition of the external solutions, in which the bentonite column immersed. The result showed that the pH value of porewater in the cementitious condition was around 9 during the immersed time 1 to 3 months, while after 6 months became the porewater pH 10.6, which was equal to pH of the external solution.
Owada, Hitoshi*; Mihara, Morihiro; *; *
JNC TN8400 2000-027, 19 Pages, 2000/08
Bactch leaching experiments of granite with the artifitial cement leachate and the leachate of low-alkalinity-cement (LW) were carried out to evaluate the effect of the hiperalkaline plume on the environment of the high-level and TRU radioactive waste repository. Dissolution of Si and Al from feldspar included in the granite and precipitation of C-S-H were confirmed from the results of the leaching experiments with artifitial cement leachate. From this result it was found that the composition of sorrounding rock changed. It also suggested that the retardation factor of migration of radionuclides would change. On the contrary, only decrease of concentrations in Si, Al and Ca in the leachate was observed in the experiment with LW. This result might indicate that C-S-H and/or C-A-S-H precipitated as secondary minerals in the LW case. From these results, it was considered that the hiperalkaline plume from the cementitious leachate might caused the change of disposal conditions such as the change in distribution coefficients of rock by precipitation of the secondary mineral and the increase in hydraulic conductivity by the dissolution of rock. On the other hand, the influences of the LW would be comparatively small, because LW and granite might equilibrate in short time.
Kato, Hiroshige*; Sato, Mitsuyoshi*; Owada, Hitoshi*; Mihara, Morihiro;
JNC TN8430 2000-008, 53 Pages, 2000/05
Cementitious materials will be used in TRU waste disposal repository. In such cases, it is considered that the migration of alkaline leachates from cementitious materials, so called high pH plume, will cause dissolution of rock and precipitation of secondary minerals. In addition, the high pH plume will move along the flow of groundwater, so it is predicted that rock formation and components of high pH groundwater vary with time and space. However, time and spatial dependence of the variations of secondary minerals and groundwater components has not been clarified. In order to acquire the data of variations of secondary minerals and groundwater components, we carried out the rock alteration experiments with column method. The crushed granodiorite was filled into 4 meters length column (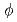 3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80
3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80 C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
Muroi, Masayuki*
JNC TJ8400 2000-042, 142 Pages, 2000/02
Hyperalkaline pore water of cementitious material used in TRU waste repository would react with bentonite and cause the increased porosity and the loss of the swelling and sorption ability. This work is a modelling study on bentonite-cement pore water. The possible extent of reaction between bentonite and cement pore water was simulated using the PRECIP reaction-transport code. Three cement pore fluid compositions (leachates 1,2 and 3) were reacted with a 1-D, 1m flowpath of bentonite (+ sand) at 25 and 70 C. Key minerals were allowed to dissolve and precipitate using kinetic reaction mechanism. Leachate 1 was the most aggressive fluid (highest pH, Na and K), and leachate 3 (1owest pH, Na and Ca) the least aggressive. Simulation with leachate 1 showed total removal of primary bentonite minerals up to 60 cm from the contact with cement after
C. Key minerals were allowed to dissolve and precipitate using kinetic reaction mechanism. Leachate 1 was the most aggressive fluid (highest pH, Na and K), and leachate 3 (1owest pH, Na and Ca) the least aggressive. Simulation with leachate 1 showed total removal of primary bentonite minerals up to 60 cm from the contact with cement after  1000 years. The maximum porosity increase observed was in leachate 1(up to 80-90%) over a narrow zone 1-2 cm. Simulations with all fluids showed total filling of pore with CSH minerals in a zone very close to the interface with the cement, whereas zeolites and sheet silicates formed far away. For a given leachate composition, there was little difference in the profiles at the two temperatures studied. It was suggested that bentonite alteration was not sensitive to the kinetic parameters over the conditions studied. The conceptual model chosen for the modelling study assumed that there was an unlimited amount of cement pore fluid available for reaction with bentonite so that the results of the simulations represent a conservative (pessimistic) estimate. There were a number of uncertainties associated with the modelling which relate to assumptions concerning: the kinetic mechanisms for dissolution and growth of minerals at elevated pH; evolving surface areas of minerals with time; thermodynamic data for CSH minerals, zeolites and aqueous species at high pH; the synergy between changing porosity and fluid ...
1000 years. The maximum porosity increase observed was in leachate 1(up to 80-90%) over a narrow zone 1-2 cm. Simulations with all fluids showed total filling of pore with CSH minerals in a zone very close to the interface with the cement, whereas zeolites and sheet silicates formed far away. For a given leachate composition, there was little difference in the profiles at the two temperatures studied. It was suggested that bentonite alteration was not sensitive to the kinetic parameters over the conditions studied. The conceptual model chosen for the modelling study assumed that there was an unlimited amount of cement pore fluid available for reaction with bentonite so that the results of the simulations represent a conservative (pessimistic) estimate. There were a number of uncertainties associated with the modelling which relate to assumptions concerning: the kinetic mechanisms for dissolution and growth of minerals at elevated pH; evolving surface areas of minerals with time; thermodynamic data for CSH minerals, zeolites and aqueous species at high pH; the synergy between changing porosity and fluid ...
Yui, Mikazu; Makino, Hitoshi; Ashida, Takashi; ; Ishiguro, Katsuhiko; Neyama, Atsushi*
PNC TN8410 92-161, 177 Pages, 1992/09
None